How to use Nernst equation
How to use Nernst equation
Table of Contents
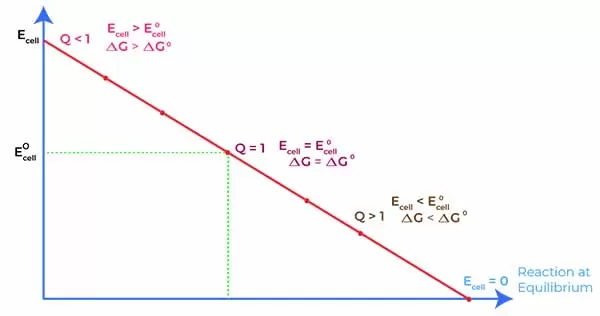
Introduction
In the realm of electrochemistry, understanding of how to use Nernst equation stands as a powerful tool that bridges the gap between thermodynamics and electrochemical cell behavior. How to effectively use this equation can enhance your proficiency in predicting the voltage of an electrochemical cell under non-standard conditions, as well as deepen your grasp of reaction spontaneity and equilibrium.
Whether you’re a student striving to ace your chemistry exams, a researcher delving into innovative energy solutions, or simply an enthusiast eager to explore the intricacies of chemical reactions, mastering the Nernst equation is essential.
In this blog post, we’ll demystify the Nernst equation, breaking down its components and providing practical examples to help you harness its full potential in your studies and experiments.
Nernst equation formula
Understanding of how to use Nernst equation is a vital tool in electrochemistry, especially when it comes to calculating cell potential under nonstandard conditions. Understanding how to use Nernst equation allows you to gain deeper insights into how electrochemical cells function in real-world scenarios, where concentrations and temperatures may vary.
The equation is elegantly simple yet remarkably powerful, making it an indispensable resource for chemists and students alike.
At its core, the Nernst equation is expressed as:
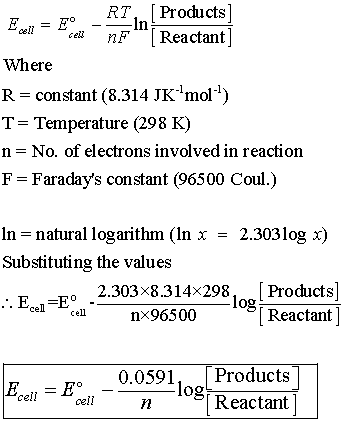
To use the Nernst equation, you first need to gather the required variables. It’s crucial to consider the specific concentrations of the reactants and products, as the equation allows you to relate these concentrations to the overall cell potential.
For instance, if you have a redox reaction with a known standard potential but are operating outside standard conditions (like different ion concentrations), the Nernst equation enables you to calculate an adjusted cell potential that reflects these changes.
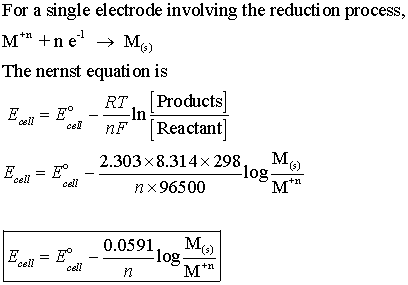
How to use Nernst equation for an electrochemical cell, having a net reaction.
x A +y B →n C + m D
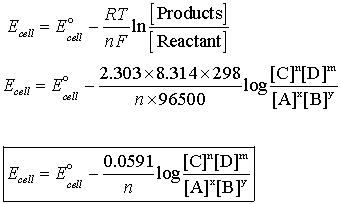
How to use Nearnst equation where the reaction is having gaseous reactant or products or both
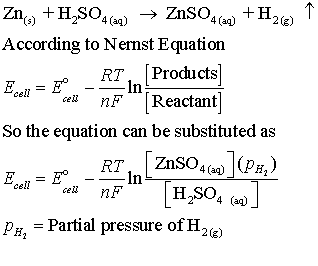
When you plug in the appropriate values for temperature and concentrations into the equation, you’ll find that as the concentration of products increases or reactants decrease, the cell potential shifts accordingly.
This dynamic nature of cell potential is key to understanding how to use Nernst equation for batteries perform under load or how various environmental factors affect electrochemical systems. By mastering how to use Nernst equation, you not only enhance your understanding of electrochemical cells but also empower yourself to anticipate how changes in the system can affect performance.
Numerical
For a following cell reaction:
Mg (s) +2 Ag+1 (0.0001M) → Mg2+ (0.13 M) + 2 Ag(s)
Calculate Ecell at 298 K Given Standard reduction potentials for Mg and Ag are -2.37 v and +0.80 V respectively
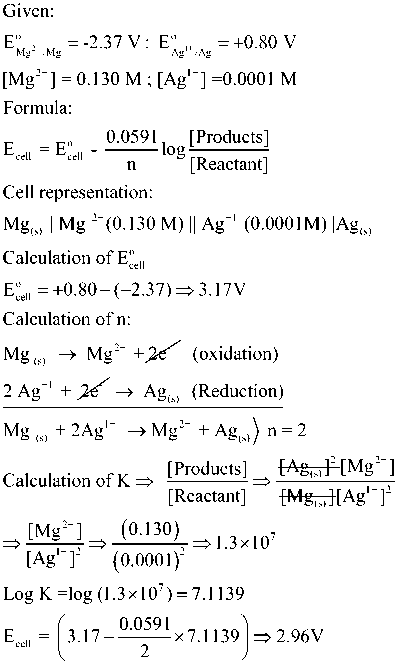
As we wrap up our exploration of how to use Nernst equation and its application in calculating cell potential under nonstandard conditions, we hope you feel empowered to tackle electrochemical problems with confidence.
Understanding how to adjust for varying concentrations, temperatures, and pressures can demystify complex reactions and lead to richer insights in your studies.
By harnessing the Nernst equation, you can gain a clearer picture of how real-world scenarios impact cell performance, paving the way for deeper learning in your chemistry journey.
If you have any further questions or want to expand your understanding, don’t hesitate to reach out or explore more resources at myetutors. Empower your academic pursuits with knowledge, and watch your grasp of chemistry flourish!
