Henry’s Law and applications 1
Table of Contents
Solubility
Henry’s law and applications are about solubility.
The solubility of the substance is the maximum amount of substance that can be dissolved in a specified amount of solvent at a given temperature and pressure.
Both temperature and pressure are important while calculating the solubility of the substance. Other than this the nature of solute and solvent are also important in deciding the solubility of the substance
Solubility of solid in liquid
Henry’s law and applications is about solubility.
when the solubility of a solid in liquid is taken into consideration then the important factors on which solubility depends are
Nature of solid:
The solids can be ionic and covalent. Ionic compounds are readily soluble in water or polar solvents this is because water is a polar solvent and the irons formed by any compounds are readily stabilized by the polarity in the polar solvents.
Thus ionic compounds or covalent compounds which have polarity are soluble in polar solvents.
But naphthalene and Sulphur are not soluble in water, but they are soluble in nonpolar solvents like carbon disulfide, and benzene.
Thus in solubility of solids in liquids can be generalized as “like dissolves like” that is polar solutes are dissolved in polar solvents and nonpolar solutes get dissolved in nonpolar solvents.
Saturated solution
A solution of gas or solid in liquid attains such a state at a given temperature and pressure that no more solute can be added into the solvent to increase the concentration of the solute is termed as the saturated state.
An unsaturated state of the solution with gas or solid is when further solute can be added at the given temperature and pressure.
Solubility of gases and liquids
Henry’s law and applications are about solubility.
The solubility of gases and liquids is largely dependent on pressure and temperature. Polarity or interaction between gas molecules and liquids also influences the solubility of the gas.
For example, oxygen gets dissolved in water but because there are very weak interactions between water molecules and oxygen gas thus solubility of oxygen gas is not very high, and generally, it is expressed in ppm.
On the other hand, hydrochloric acid gas is readily soluble in water because of polarity over the HCl molecule.
Effect of pressure on the solubility of gas
Henry’s law and applications are about the solubility of gases.
The solubility of gases increases with increasing pressure. When gases are in contact with liquid at a given pressure according to Le-Chatterler’s principle, at equilibrium, dynamic state, the molecules of the liquid phase and gases phase are in equilibrium.
When the pressure has increased the molecules from the gases phase move into the liquid phase because according to Le-Chatterler’s principle with increasing pressure, the system will try to decrease the number of gas molecules and gas molecules will decrease when they enter into the liquid phase so more gas particles will enter into liquid phase thus the concentration of gas molecules in liquid would increase.
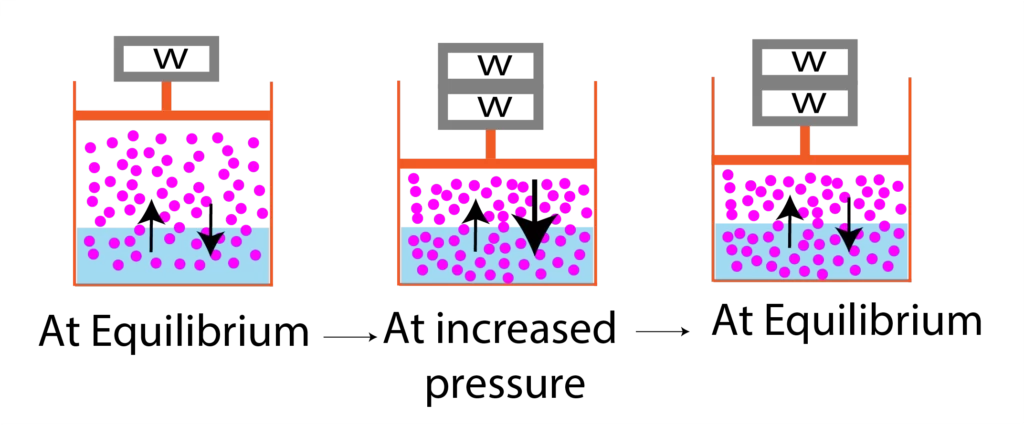
Henry’s Law:
Henry’s law and applications are about the solubility of the gas in the liquid.
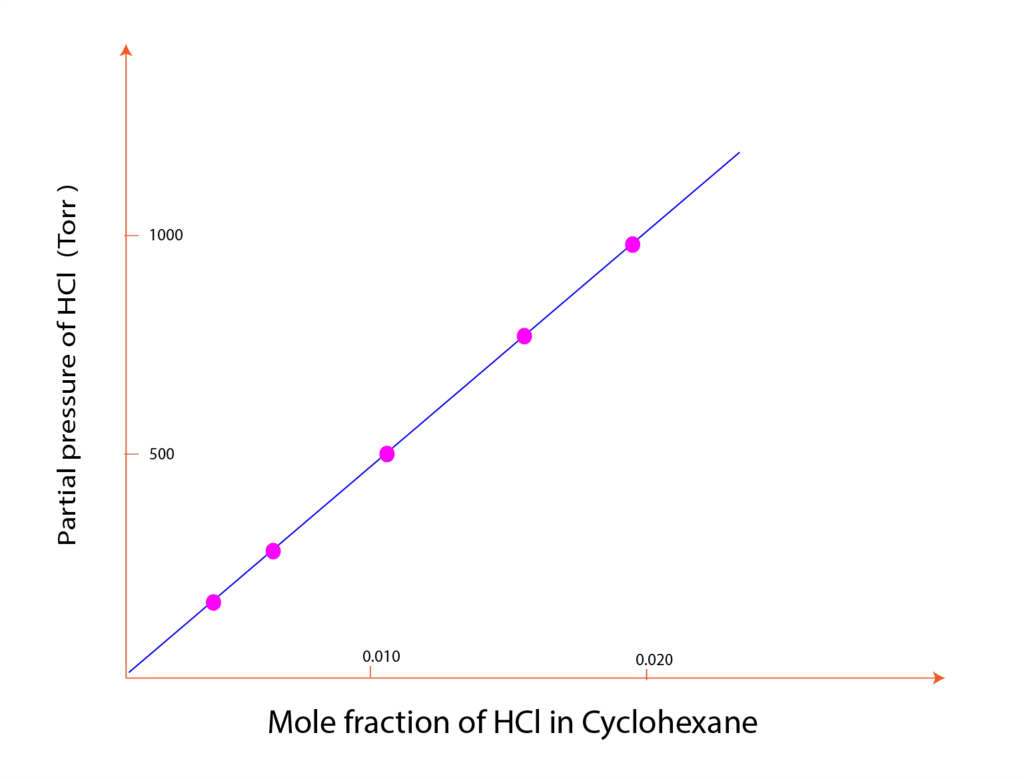
It is a quantitative relationship between the pressure and solubility of the gas. According to this law, the solubility of the gas is directly proportional to the partial pressure of the gas over the liquid or solution.
Dalton independently concluded that the mole fraction (X) of the gas in the solution is directly proportional to the partial pressure (p) of the gas over the solution.
The statement of Henry’s law is the partial pressure of the gas in the vapor phase is directly proportional to the mole fraction of the gas in the solution. Mathematically the relationship can be expressed as (X)

Application of Henry's Law
Henry’s law finds several applications in industry and explains some biological phenomena. Some important applications are:
To increase the solubility of carbon dioxide in soft drinks and soda water, the bottle is sealed under high pressure.
Scuba divers must cope with high concentrations of dissolved gases while breathing air at high pressure underwater. When the divers come toward the surface, the pressure gradually decreases. This releases the dissolved gases and leads to the formation of bubbles of nitrogen in the blood.
This blocks capillaries and creates a medical condition known as bends, which are painful and dangerous for life, to avoid bends and the toxic effects of high concentrations of nitrogen in the blood, the tanks used by scuba divers are filled with air diluted with helium (11.7% helium, 56.2% nitrogen and 32.1% oxygen).
At high altitudes, the partial pressure of oxygen is less than that at the ground level. This leads to low concentrations of oxygen in the blood and tissues of people living at high altitudes or climbers. Low blood oxygen causes climbers to become weak and unable to think clearly, symptoms of a condition known as anoxia.
You may like
Become a Member
Purchase a membership for expert guidance tailored for IIT, NEET, and CBSE exams. Unlock your potential and ace your exams with personalized tutoring and resources!
Purchase YouTube Channel
Watch Our content tailored to help you excel in JEE Mains and NEET-UG. With expert tips, educational videos, and insights, you’ll be equipped with the knowledge and strategies you need to succeed in your exams
Join Channel Telegram Channel
Join our Telegram channel to unlock a treasure trove of practice papers tailored for IIT, NEET, and CBSE exams. Stay ahead of the curve and ace your tests with confidence!
Join Channel 