Colligative property 4
Table of Contents
Colligative Property 4
Introduction to Osmotic Pressure
When it comes to mastering the nuances of chemistry, understanding colligative properties 4 is essential for CBSE examinations, and one of the most intriguing aspects is osmotic pressure.
As students delve into the world of solutions, they’ll discover that adding a solute into a solvent doesn’t just change the concentration—it unlocks a host of fascinating behaviors and principles that govern how substances interact at a molecular level.
In this blog post, we will unpack the intricacies of osmotic pressure, guiding you through the fundamental concepts with clarity and insight.
Whether you’re preparing for exams or simply looking to deepen your comprehension, we’ll provide you with the knowledge and strategies you need to excel in your studies with myetutors.
Join us as we explore the incredible role that solutes play in shaping the properties of solutions and prepare to impress in your upcoming chemistry assessments!
Introduction: Understanding Colligative Property 4 in Chemistry
Colligative properties play a crucial role in chemistry, particularly in understanding the behavior of solutions.
For CBSE students preparing for examinations, grasping these concepts can enhance their overall understanding of physical chemistry.
This blog post will focus specifically on osmotic pressure, one of the key colligative properties, and its implications when adding solute into a solvent.
What are Colligative Properties?
Before delving into osmotic pressure, it’s essential to define colligative property 4. These are properties of solutions that depend on the number of solute particles in a given amount of solvent rather than the identity of the solute.
The main colligative properties include vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure.
Defining Osmotic Pressure
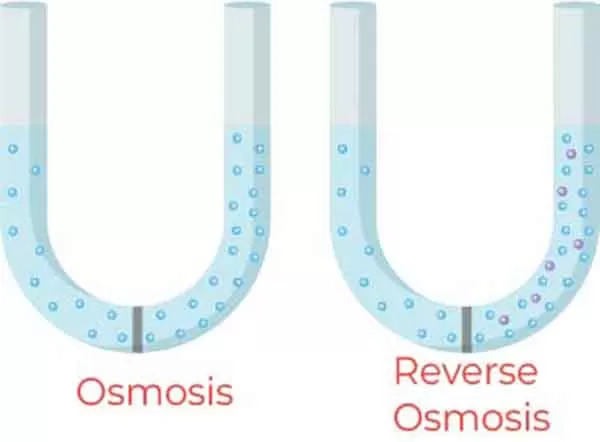
Osmotic pressure is the pressure required to stop the flow of solvent into a solution through a semipermeable membrane.
This property is significant in various biological and chemical processes. It is directly related to the concentration of solute particles in the solution, making it an important concept for students.
How to Calculate Osmotic Pressure
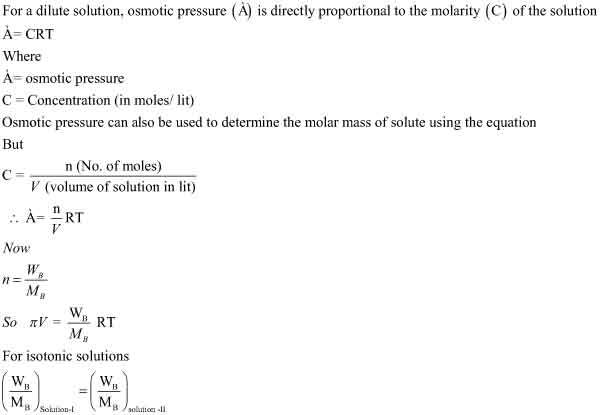
The Effect of Adding Solute to Solvent
When a solute is added to a solvent, several changes occur:
– An increase in the number of solute particles leads to a rise in osmotic pressure.
– The physical properties of the solution change, affecting its boiling and freezing points.
– Students should experiment with practical examples to see how varying concentrations impact osmotic pressure.
Practical Applications of Osmotic Pressure
Osmotic pressure has several real-world applications:
– Biological systems, such as the function of cells and the transport of nutrients and waste.
– Industrial applications where controlling solute concentrations is vital (e.g., food preservation, chemical syntheses).
– Understanding osmotic pressure can also aid in grasping other related topics such as tonicity and physiological effects of solutions on cells.
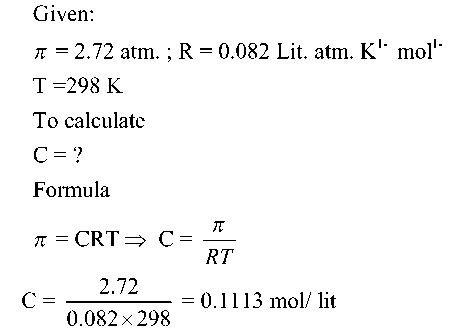
Numerical
an aqueous solution of glucose (C6H12O6) has an osmotic pressure of 2.72 atmosphere at 298 K. How many moles of glucose is dissolved per litre of solution? (R =0.082 Lit. atm. K1- mol1-)
Tips for CBSE Students
Practice Problems: Work on calculations involving different scenarios of solute and solvent combinations to strengthen your understanding.
Visual Learning: Use diagrams to visualize the semipermeable membrane and the movement of solvent and solute particles.
Group Study: Discussing these concepts with classmates can help reinforce your learning through different perspectives and explanations.
Mastering Osmotic Pressure for Success in Exams
Understanding colligative property 4, particularly osmotic pressure, is essential for CBSE examinations. With proper grasp and application of the formulas and concepts discussed, students can effectively tackle related questions and excel in their chemistry assessments.
Remember, practice makes perfect, so utilize resources like Myetutors to clarify doubts and strengthen your foundation in chemistry.
