Colligative property 3
Colligative Property 3
Table of Contents
Introduction
Understanding the intricacies of chemistry is essential for students preparing for their CBSE examinations, and one of the fascinating concepts to grasp is colligative properties, particularly the depression of the freezing point when a solute is added to a solvent.
This phenomenon showcases the relationship between solute concentration and the behavior of solvents, shedding light on why adding salt to icy roads can prevent freezing.
In this comprehensive blog post, we will delve into the principles of colligative properties, explore the scientific underpinnings of freezing point depression, and provide practical examples to clarify these concepts.
Whether you’re looking to ace your exams or simply intrigued by the wonders of chemistry, this guide from Myetutors will equip you with the knowledge to navigate this essential topic confidently.
Introduction to Colligative Property 3
Colligative properties are vital concepts in chemistry that rely on the number of solute particles in a solution rather than the identity of those particles.
This blog post will delve into one specific colligative property 3-the depression of the freezing point.
Understanding this concept is crucial for CBSE examinations, especially when dealing with solutions in chemistry.
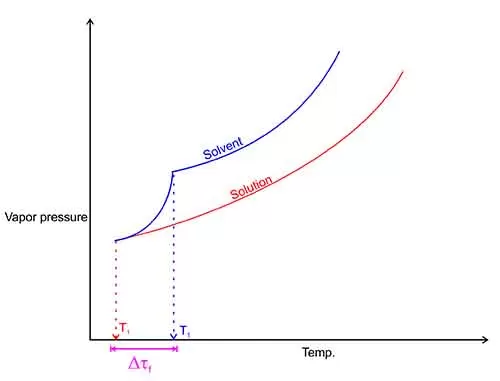
What is Freezing Point Depression?
Freezing point depression occurs when a solute is added to a solvent, leading to a decrease in the solvent’s freezing point.
This phenomenon is important in various applications, such as antifreeze solutions in vehicles and the culinary arts. Knowing how and why this occurs provides a foundational understanding of solution chemistry.
How Does Adding Solute Affect Freezing Point?
When a solute is added to a solvent, the solute particles disrupt the orderly arrangement of the solvent molecules.
This disruption requires a lower temperature for the solvent molecules to come together and form a solid (ice).
The extent of freezing point depression depends on the concentration of the solute.
The Formula for Calculating Freezing Point Depression

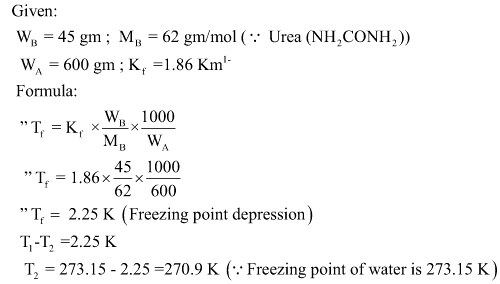
Numerical
45 gm of Urea is dissolved in 600 gm of water calculate
- Freezing point depression
- Freezing point of solution
Kf for water is 1.86 Km1-
Example Scenario: Salt in Water
Consider the example of adding table salt (NaCl) to water. Salt dissociates into sodium (Na⁺) and chloride ions (Cl⁻), which means the van ‘t Hoff factor( i) would be 2.
Understanding how this value influences the freezing point depression can enlighten students about real-world applications, such as road safety in winter by using salt on icy roads.
Importance of Colligative Properties in Everyday Life
Colligative properties, including freezing point depression, have significant applications in various fields from food science to chemical engineering.
For example, knowing how and why antifreeze works in automobiles can sustain vehicle performance in cold weather.
Such practical applications can heighten interest and understanding when studying these concepts for the CBSE examinations.
