Colligative property 1
Colligative Property 1
Table of Contents
Colligative Property 1
Understanding the chemistry behind solutions generates curiosity and opens up a world of fascinating interactions and properties, particularly when it comes to the concept of colligative properties 1.
These unique characteristics arise when a solute is mixed with a solvent, leading to intriguing effects that depend on the number of solute particles rather than their identity.
For students navigating the complexities of solution chemistry or anyone eager to grasp how everyday substances behave at the molecular level, the study of colligative properties is essential. In this blog post, we’ll delve into the key colligative properties—such as Relative lowering in vapor pressure, and osmotic pressure—and explore how they apply to various solutions, ensuring you have a solid foundation in this critical area of chemistry.
Whether you’re preparing for an exam or simply curious about the science that underpins our world, this guide will illuminate the intriguing results of mixing solutes and solvents.
Typers of colligative properties
The properties of solution which depends on only the number of solute particles but not on the nature of solute are called colligative properties.
Types of colligative properties: There are four colligative properties namely,
- Relative lowering of vapor pressure
- Elevation of boiling point
- Depression of freezing point
- Osmotic pressure
In this blog post I am discussing i) and iv) colligative property 1
Relative Lowering in Vapor Pressure
Relative lowering in vapor pressure is a fundamental concept in the study of colligative property 1, which are properties of solutions that depend largely on the number of solute particles rather than their identity.
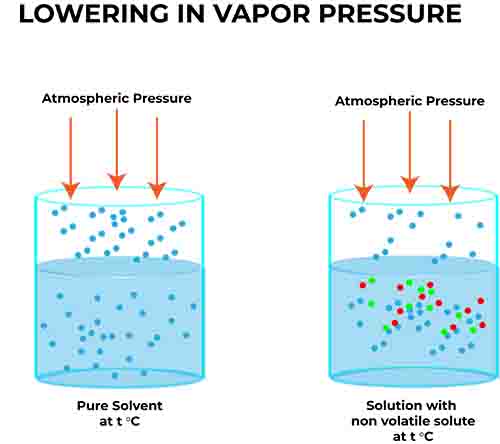
When a non-volatile solute is dissolved in a solvent, the vapor pressure of the solvent is lowered compared to that of the pure solvent. This reduction in vapor pressure is particularly significant as it illustrates how the presence of solute particles alters the escape tendency of the solvent molecules.
To understand this concept of colligative property 1, consider a pure solvent at equilibrium, where molecules are transitioning between the liquid and vapor phases. When a solute is introduced, it occupies space at the surface of the liquid, which decreases the number of solvent molecules that can escape into the vapor phase. As a result, while the solvent’s vapor pressure is lowered, the concentration of the solute particles becomes paramount in quantifying this effect.
This phenomenon can be expressed mathematically using Raoult’s Law, which states that the relative lowering in vapor pressure colligative property 1 is equal to the mole fraction of the solute.
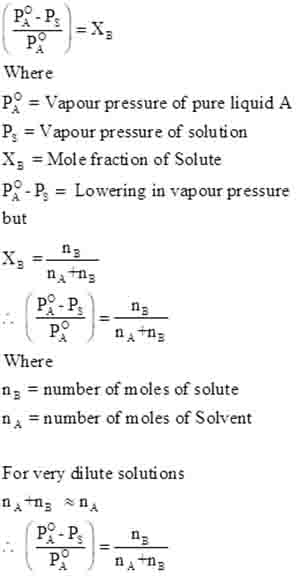
For instance, if you dissolve a certain amount of salt into water, the vapor pressure of the water will decrease proportionately to the number of salt particles in the solution.
This key principle has important practical applications, from understanding how salt affects the freezing of roads in winter to the functionality of various chemical processes in laboratories and industry.
Understanding the relative lowering of vapor pressure not only gives insights into the nature of the solution but also provides a foundational concept for diving deeper into colligative properties and their implications in real-world scenarios.
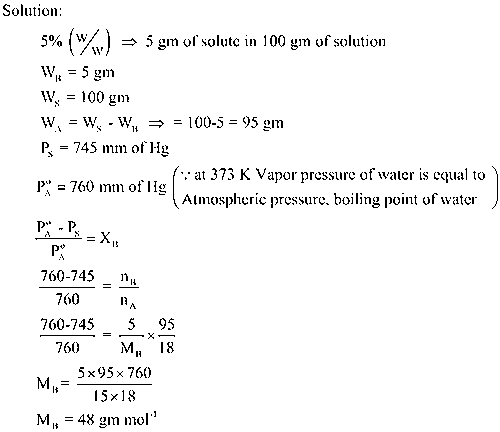
Numerical
The vapor pressure of 5% aqueous solution of a non volatile organic substance at 373 K is 745 mm of Hg. Calculate the molecular mass of organic substance (Solute)